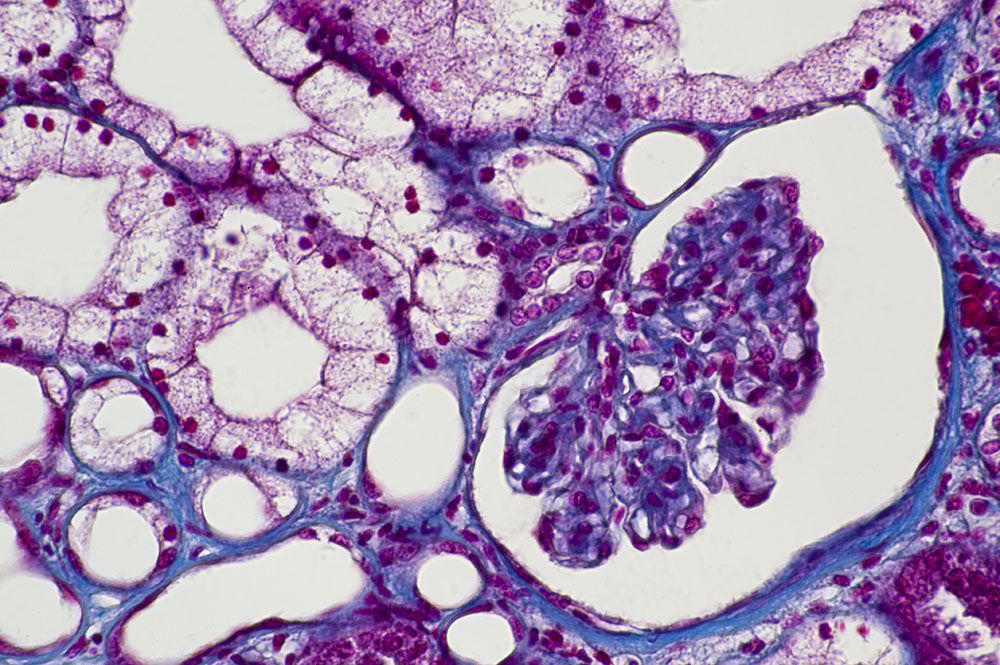
Imaging blood flow through cell-free kidneys
An advanced imaging technique is providing insights into organ scaffold viability, which could help reduce donor organ shortages in future.
Research at Khalifa University has imaged blood flowing through a kidney without its cells for the first time; an important step towards the development of bioartificial organs for research or transplant. The research was made possible thanks to the innovative technique of intravital microscopy, which enables the imaging of the internal processes inside living animals.
This pioneering study, conducted in rats, harnessed an innovative combination of whole organ decellularization, transplantation and intravital microscopy. It showed how the blood could flow without clotting, despite being exposed to collagen inside the cell-less kidney. Organs divested of their internal cells in this way, commonly referred to as scaffolds, are a popular option in bioengineering research, as they can then be filled with cells from a specific patient.
“Remarkably, this thing can actually withstand blood flow,” says Peter Corridon, a kidney specialist at the university’s College of Medicine and Health Sciences, who carried out the new research. “So, it gives us insight into how we might be able to build a better, stronger, or more resilient structure as we repopulate the scaffold with cells.”
The findings, published in Scientific Reports, indicate that the advanced imaging technique “may provide novel insight into scaffold viability and identify ways to promote scaffold longevity and vasculogenesis in whole decellularized organs and help reduce donor organ shortages within the foreseeable future.”
In approved experiments, Corridon removed kidneys from rats, eliminated the internal cells from the organs, and then transplanted the scaffolds back into the animals. These kidney scaffolds were not empty or hollow; they retained their internal structure of blood vessels. But with no cells, the blood that entered the kidney scaffold was exposed to collagen, which would usually be expected to trigger the blood-clotting process.
“Technically speaking, when you have this scaffold and you have blood flowing, it should clot, it should not work whatsoever,” says Corridon. “And oddly enough, this thing flowed for days.” After that, the clotting process did kick in and the internal structure of the kidney scaffold broke down.
The results are important for two reasons. First, they show that blood could continue to flow while cells begin to regrow inside a kidney scaffold. Second, as the blood flows it could carry with it the stem cells and other components needed to help the kidney cells grow and form tissue.
“It was painstaking work,” Corridon says, adding that there remains some way to go before the development of a fully artificial organ. “The kidney has about 50 cell types. And it’s hard to get every cell and every structure right to create all these complex systems,” he explains.
Reference
- Corridon, P.R. Capturing effects of blood flow on the transplanted decellularized nephron with intravital microscopy. Scientific Reports 13, 5289 (2023). | Article